Pharmaceutical Compliance Translation: Precision for Global Regulatory Success

Pharmaceutical Compliance Translation: Precision for Global Regulatory Success Pharmaceutical compliance translation ensures that regulatory documentation meets the exact requirements of health authorities across multiple jurisdictions. Pharmaceutical companies operating in the EU, US, and other regulated markets face stringent translation requirements that directly affect product approval timelines and market access.
EMA Submission Translation: Navigating EU Pharmaceutical Regulatory Requirements
EMA Submission Translation: Navigating EU Pharmaceutical Regulatory Requirements EMA submission translation requires precision, regulatory expertise, and strict adherence to the European Medicines Agency’s Quality Review of Documents (QRD) templates. Pharmaceutical companies seeking marketing authorisation through the Centralised Procedure must translate product information into all official EU languages within demanding timelines—often just five days post-opinion. This […]
Drug Labeling Translation: Precision for Pharmaceutical Compliance
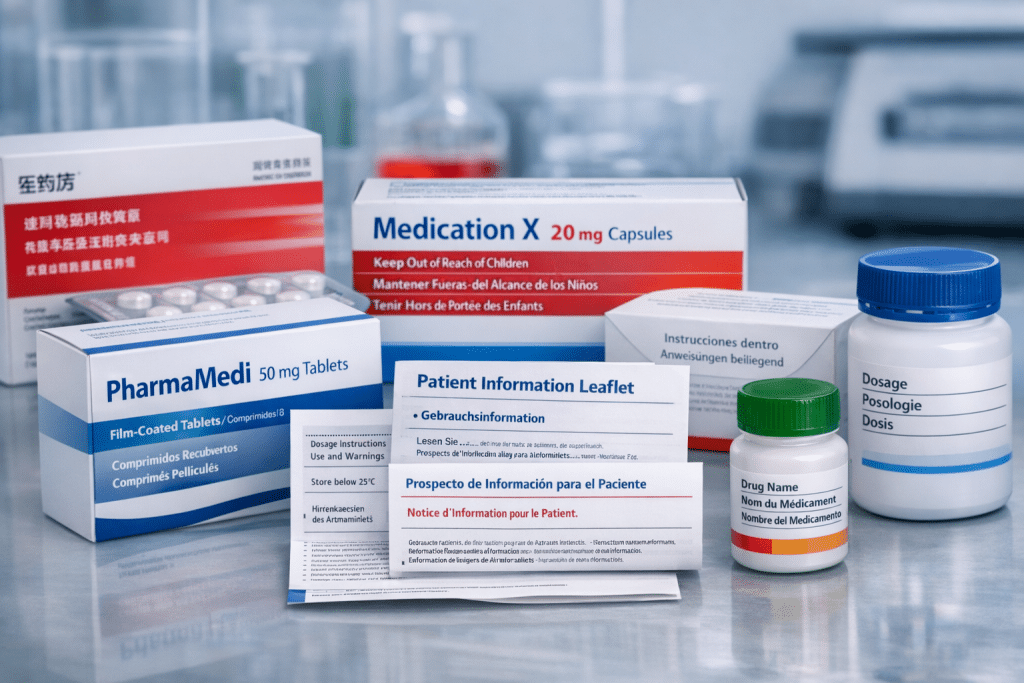
Drug Labeling Translation: Precision for Pharmaceutical Compliance Drug labeling translation converts pharmaceutical product information into target languages while preserving regulatory accuracy. When a medication reaches international markets, every word on the package insert, carton text, and patient leaflet carries legal and safety implications that require specialized translation expertise.
Clinical Documentation Translation: Precision for Global Trial Success

Clinical Documentation Translation: Precision for Global Trial Success Clinical documentation translation converts trial protocols, informed consent forms, and safety reports into target languages while preserving scientific accuracy and regulatory compliance. Pharmaceutical sponsors conducting multi-country studies require translations that meet ICH GCP standards and satisfy ethics committee requirements across diverse jurisdictions.
Pharma Regulatory Translation: Ensuring Compliance for Global Market Authorization

Pharma Regulatory Translation: Ensuring Compliance for Global Market Authorization Pharma regulatory translation converts critical drug registration documentation to meet the stringent requirements of authorities like the EMA, FDA, and national health agencies. Companies seeking marketing authorization across multiple jurisdictions depend on translation accuracy to avoid delays, queries, and potential rejection of their submissions.
Pharmaceutical Translation Services: Ensuring Regulatory Compliance Across Global Markets

Pharmaceutical Translation Services: Ensuring Regulatory Compliance Across Global Markets Pharmaceutical translation services convert complex regulatory, clinical, and scientific documentation while maintaining the precision required for global drug approvals. When patient safety and market authorization depend on accurate multilingual documentation, specialized translators with pharmaceutical expertise become essential partners in your regulatory strategy.

